Chrysidids are parasites of other insects, or more parasitoids, which means that their activity - in most cases - brings death to their hosts; some species are also cleptoparasites, which means that they use the food carried on by the host as resources for their larvas.

Chrysidid females own a long telescopic ovipositor that acts as an instrument to place the egg inside the nest of the host wasp. Such ovipositor derives from the evolutive reduction of a vulnerant apparatus (sting) to an introflected apparatus (segments inside the abdomen).
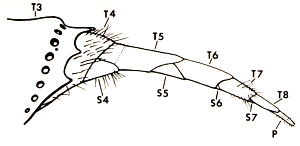
Ovipositor: TN = gastral terga; SN = gastral sterna (from Morgan (1984)).
For some Chrysidid species, the specialization in the choice of the host is high and determines a strong host/parasite association. For other species, instead, the choice of the host seems to be determined by the type of nest constructed by the host and for which the Chrysidid "is trained". The nature of the primary food source is a feature which distinguishes the subfamilies: Amiseginae and Loboscelidiinae feed on Phasmid eggs; Chrysidinae (except for the genus Praestochrysis, host of Lepidoptera) and Parnopinaefeed on larvas of other Hymenoptera (Eumenids, Sphecids, Apoids, Vespoids, Tenthredinids).
 Cleptinae prey on prepupal Tenthredinoidea. Thanks to the studies of Clausen (1940), Gauss (1964) and Dahlsten (1961 and 1967) we can make some generalizations. Adult Cleptes search for their hosts' cocoons in the soil or in the ground; once the cocoon is localized, the female Cleptes open a hole in the wall with their jaws, then they insert their long ovipositor and put their egg on the host larva. Once the ovideposition has taken place, they close the hole with a mucillaginous material; the Chrysidid larva, after having consumed the host, will secrete its own cocoon inside the host's cocoon.
Cleptinae prey on prepupal Tenthredinoidea. Thanks to the studies of Clausen (1940), Gauss (1964) and Dahlsten (1961 and 1967) we can make some generalizations. Adult Cleptes search for their hosts' cocoons in the soil or in the ground; once the cocoon is localized, the female Cleptes open a hole in the wall with their jaws, then they insert their long ovipositor and put their egg on the host larva. Once the ovideposition has taken place, they close the hole with a mucillaginous material; the Chrysidid larva, after having consumed the host, will secrete its own cocoon inside the host's cocoon.
 Identical modalities are observed in Chrysidinae of the Genus Praestochrysis. Piel (1933) studied the biology of Praestochrysis shanghaiensis, parasite of the nocturnal butterfly Monema flavescens Walker (Lepidoptera Limacodidae). The Chrysidid attacks the silky cocoon of the caterpillar as soon as it has been hardened; with some bites, it produces a hole wide enough to let the ovipositor enter inside. Once this operation is completed, the chrysidid female abrades the material around the cocoon and pastes it back with her saliva in order to close the hole. It has been experimentally observed that if the hole fails to be closed, the entire content of the cocoon is destroyed by molds.
Identical modalities are observed in Chrysidinae of the Genus Praestochrysis. Piel (1933) studied the biology of Praestochrysis shanghaiensis, parasite of the nocturnal butterfly Monema flavescens Walker (Lepidoptera Limacodidae). The Chrysidid attacks the silky cocoon of the caterpillar as soon as it has been hardened; with some bites, it produces a hole wide enough to let the ovipositor enter inside. Once this operation is completed, the chrysidid female abrades the material around the cocoon and pastes it back with her saliva in order to close the hole. It has been experimentally observed that if the hole fails to be closed, the entire content of the cocoon is destroyed by molds.
The female Chrysidinae generally penetrate the nest of the host during its construction and place their egg in a hidden spot of the cell. Some Chrysidids, like Stilbum cyanurum, seem to be specialized in preying on different species that construct their nests with mud (like Sceliphron sphecids). Other species of Chrysidids are more taxa-specific and prey only on certain Genera or just on single species.
 There are two basic strategies in parasitizing hosts. The first one wants that the Chrysidid starts with eating the host egg or the young host larva and then eats the food resources present in the nest (cleptoparasitism); the second one wants that the Chrysidid waits for the development of the host larva to its prepupal stadium, and then the Chrysidid kills it after cleaning the nest. This second way generally happens when the host belongs to those apoids who accumulate pollen and other sweets in the nest, impossible to be synthetized by Chrysidids. When the supplies accumulated by the the mother wasp for her larva are enough to feed also the parasite larva, it is possible to assist to the development of both the larvae without any trace of parasitism. In some cases, it is possible to see more than one Chrysidid specimen from a single cell, rather than a single Chrysidid from the single cell as it generally happens. These facts, underlined by Kimsey & Bohart (1990), could be explained as a behaviour of parsimony and of optimization of the available resources, when sufficient, avoiding the energy-expensive parasitism.
There are two basic strategies in parasitizing hosts. The first one wants that the Chrysidid starts with eating the host egg or the young host larva and then eats the food resources present in the nest (cleptoparasitism); the second one wants that the Chrysidid waits for the development of the host larva to its prepupal stadium, and then the Chrysidid kills it after cleaning the nest. This second way generally happens when the host belongs to those apoids who accumulate pollen and other sweets in the nest, impossible to be synthetized by Chrysidids. When the supplies accumulated by the the mother wasp for her larva are enough to feed also the parasite larva, it is possible to assist to the development of both the larvae without any trace of parasitism. In some cases, it is possible to see more than one Chrysidid specimen from a single cell, rather than a single Chrysidid from the single cell as it generally happens. These facts, underlined by Kimsey & Bohart (1990), could be explained as a behaviour of parsimony and of optimization of the available resources, when sufficient, avoiding the energy-expensive parasitism.
The Chrysidids parasitoids of potentially vulnerant wasps (sting, jaws) show a morphologic-functional adaptation of defensive nature: the abdominal segments - strongly sclerotized on the external surface and concave in the ventral surface - allow the lodging of antennas and legs when the Chrysidid closes itself into a defensive sphere. Such a behaviour prevents the host from mutilating or from stinging the Chrysidid.
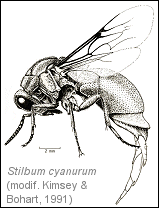 Móczár (1961) has reported some observations on the Stilbum cyanurum species, which parasites the mud nests of the Sceliphron destillatorium (Illiger) sphecid wasp. The female wets a point of the dry mud of the nest with a drop coming from her mouth parts and then touches it with her ovipositor. The operation, repeated a few times, brings to the penetration of the muddy wall and to the deposition of an egg into the cocoon of the Sceliphron. The Chrysidid ovipositor is very strong and indented, so it can be used to work like a knife. After the ovideposition and the extraction of the ovipositor, the wet mud is used to close the hole, leaving a visible depression on the cell wall. Berland & Bernard (1938) have listed many hosts for Stilbum cyanurum: Sceliphron, Eumenes, Chalicodoma and Megachile, all producers of mud nests.
Móczár (1961) has reported some observations on the Stilbum cyanurum species, which parasites the mud nests of the Sceliphron destillatorium (Illiger) sphecid wasp. The female wets a point of the dry mud of the nest with a drop coming from her mouth parts and then touches it with her ovipositor. The operation, repeated a few times, brings to the penetration of the muddy wall and to the deposition of an egg into the cocoon of the Sceliphron. The Chrysidid ovipositor is very strong and indented, so it can be used to work like a knife. After the ovideposition and the extraction of the ovipositor, the wet mud is used to close the hole, leaving a visible depression on the cell wall. Berland & Bernard (1938) have listed many hosts for Stilbum cyanurum: Sceliphron, Eumenes, Chalicodoma and Megachile, all producers of mud nests.
 Carrillo & Caltagirone (1970) have made detailed observations on the host-parasite relations between two sphecid species, Solierella peckhami (Ashmead) and S. plenoculoides Fox, and the Chrysidid Pseudolopyga carrilloi. Thanks to their studies, carried out in California and in laboratory, it turns out that the female Chrysidid places the egg on the living larva of the first or of the second stage of a Hemipteran bug of the Genus Nysius (Hemiptera Lygaeidae, two species being involved: N. raphanus and N. tenellus). The two species of Solierella use paralized Nysius larvas in order to provision the nest, 4-10 larvas per cell. In such a complicated way the Chrysidid is able to make its egg enter the nest of the host without being seen and without the risk of the adult host noticing its presence and destroying its egg. It is noticeable that the egg will develop only in the case that the larva that carries it is captured and paralyzed by the Solierella. That's one is the only known case of a Chrysidid linking its egg just on a free host, which will be used in a second time as a prey by a sphecid. An interesting case of competition against the Pseudolopyga comes from a sympatric species, Hedychridium solierellae, which parasites the same species of Solierella and the Pseudolopyga itself. This Chrysidid directly places its egg in the Solierella cell and its larva feeds on the host larva, on its supplies and also on the egg or on the larva of Pseudolopyga, when present.
Carrillo & Caltagirone (1970) have made detailed observations on the host-parasite relations between two sphecid species, Solierella peckhami (Ashmead) and S. plenoculoides Fox, and the Chrysidid Pseudolopyga carrilloi. Thanks to their studies, carried out in California and in laboratory, it turns out that the female Chrysidid places the egg on the living larva of the first or of the second stage of a Hemipteran bug of the Genus Nysius (Hemiptera Lygaeidae, two species being involved: N. raphanus and N. tenellus). The two species of Solierella use paralized Nysius larvas in order to provision the nest, 4-10 larvas per cell. In such a complicated way the Chrysidid is able to make its egg enter the nest of the host without being seen and without the risk of the adult host noticing its presence and destroying its egg. It is noticeable that the egg will develop only in the case that the larva that carries it is captured and paralyzed by the Solierella. That's one is the only known case of a Chrysidid linking its egg just on a free host, which will be used in a second time as a prey by a sphecid. An interesting case of competition against the Pseudolopyga comes from a sympatric species, Hedychridium solierellae, which parasites the same species of Solierella and the Pseudolopyga itself. This Chrysidid directly places its egg in the Solierella cell and its larva feeds on the host larva, on its supplies and also on the egg or on the larva of Pseudolopyga, when present.
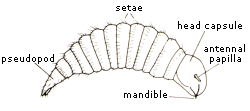
Chrysidid larva (from Morgan (1984)).






 The procedure of identification of a specimen is executed by a specialist who receives the material by a collector. After some time, he returns the material and keeps some specimens for his own collection, as an "honorarium" for his performance. The identification is performed using a
The procedure of identification of a specimen is executed by a specialist who receives the material by a collector. After some time, he returns the material and keeps some specimens for his own collection, as an "honorarium" for his performance. The identification is performed using a 
 The Loboscelidiini and the Allocoeliini lack any metallic coloration; their colors are brown, black, reddish and, in the some cases, white.
The Loboscelidiini and the Allocoeliini lack any metallic coloration; their colors are brown, black, reddish and, in the some cases, white.

 Cleptinae prey on prepupal Tenthredinoidea. Thanks to the studies of Clausen (1940), Gauss (1964) and Dahlsten (1961 and 1967) we can make some generalizations. Adult Cleptes search for their hosts' cocoons in the soil or in the ground; once the cocoon is localized, the female Cleptes open a hole in the wall with their jaws, then they insert their long ovipositor and put their egg on the host larva. Once the ovideposition has taken place, they close the hole with a mucillaginous material; the Chrysidid larva, after having consumed the host, will secrete its own cocoon inside the host's cocoon.
Cleptinae prey on prepupal Tenthredinoidea. Thanks to the studies of Clausen (1940), Gauss (1964) and Dahlsten (1961 and 1967) we can make some generalizations. Adult Cleptes search for their hosts' cocoons in the soil or in the ground; once the cocoon is localized, the female Cleptes open a hole in the wall with their jaws, then they insert their long ovipositor and put their egg on the host larva. Once the ovideposition has taken place, they close the hole with a mucillaginous material; the Chrysidid larva, after having consumed the host, will secrete its own cocoon inside the host's cocoon. Identical modalities are observed in Chrysidinae of the Genus Praestochrysis. Piel (1933) studied the biology of Praestochrysis shanghaiensis, parasite of the nocturnal butterfly Monema flavescens Walker (Lepidoptera Limacodidae). The Chrysidid attacks the silky cocoon of the caterpillar as soon as it has been hardened; with some bites, it produces a hole wide enough to let the ovipositor enter inside. Once this operation is completed, the chrysidid female abrades the material around the cocoon and pastes it back with her saliva in order to close the hole. It has been experimentally observed that if the hole fails to be closed, the entire content of the cocoon is destroyed by molds.
Identical modalities are observed in Chrysidinae of the Genus Praestochrysis. Piel (1933) studied the biology of Praestochrysis shanghaiensis, parasite of the nocturnal butterfly Monema flavescens Walker (Lepidoptera Limacodidae). The Chrysidid attacks the silky cocoon of the caterpillar as soon as it has been hardened; with some bites, it produces a hole wide enough to let the ovipositor enter inside. Once this operation is completed, the chrysidid female abrades the material around the cocoon and pastes it back with her saliva in order to close the hole. It has been experimentally observed that if the hole fails to be closed, the entire content of the cocoon is destroyed by molds. There are two basic strategies in parasitizing hosts. The first one wants that the Chrysidid starts with eating the host egg or the young host larva and then eats the food resources present in the nest (cleptoparasitism); the second one wants that the Chrysidid waits for the development of the host larva to its prepupal stadium, and then the Chrysidid kills it after cleaning the nest. This second way generally happens when the host belongs to those apoids who accumulate pollen and other sweets in the nest, impossible to be synthetized by Chrysidids. When the supplies accumulated by the the mother wasp for her larva are enough to feed also the parasite larva, it is possible to assist to the development of both the larvae without any trace of parasitism. In some cases, it is possible to see more than one Chrysidid specimen from a single cell, rather than a single Chrysidid from the single cell as it generally happens. These facts, underlined by Kimsey & Bohart (1990), could be explained as a behaviour of parsimony and of optimization of the available resources, when sufficient, avoiding the energy-expensive parasitism.
There are two basic strategies in parasitizing hosts. The first one wants that the Chrysidid starts with eating the host egg or the young host larva and then eats the food resources present in the nest (cleptoparasitism); the second one wants that the Chrysidid waits for the development of the host larva to its prepupal stadium, and then the Chrysidid kills it after cleaning the nest. This second way generally happens when the host belongs to those apoids who accumulate pollen and other sweets in the nest, impossible to be synthetized by Chrysidids. When the supplies accumulated by the the mother wasp for her larva are enough to feed also the parasite larva, it is possible to assist to the development of both the larvae without any trace of parasitism. In some cases, it is possible to see more than one Chrysidid specimen from a single cell, rather than a single Chrysidid from the single cell as it generally happens. These facts, underlined by Kimsey & Bohart (1990), could be explained as a behaviour of parsimony and of optimization of the available resources, when sufficient, avoiding the energy-expensive parasitism. Móczár (1961) has reported some observations on the Stilbum cyanurum species, which parasites the mud nests of the Sceliphron destillatorium (Illiger) sphecid wasp. The female wets a point of the dry mud of the nest with a drop coming from her mouth parts and then touches it with her ovipositor. The operation, repeated a few times, brings to the penetration of the muddy wall and to the deposition of an egg into the cocoon of the Sceliphron. The Chrysidid ovipositor is very strong and indented, so it can be used to work like a knife. After the ovideposition and the extraction of the ovipositor, the wet mud is used to close the hole, leaving a visible depression on the cell wall. Berland & Bernard (1938) have listed many hosts for Stilbum cyanurum: Sceliphron, Eumenes, Chalicodoma and Megachile, all producers of mud nests.
Móczár (1961) has reported some observations on the Stilbum cyanurum species, which parasites the mud nests of the Sceliphron destillatorium (Illiger) sphecid wasp. The female wets a point of the dry mud of the nest with a drop coming from her mouth parts and then touches it with her ovipositor. The operation, repeated a few times, brings to the penetration of the muddy wall and to the deposition of an egg into the cocoon of the Sceliphron. The Chrysidid ovipositor is very strong and indented, so it can be used to work like a knife. After the ovideposition and the extraction of the ovipositor, the wet mud is used to close the hole, leaving a visible depression on the cell wall. Berland & Bernard (1938) have listed many hosts for Stilbum cyanurum: Sceliphron, Eumenes, Chalicodoma and Megachile, all producers of mud nests. Carrillo & Caltagirone (1970) have made detailed observations on the host-parasite relations between two sphecid species, Solierella peckhami (Ashmead) and S. plenoculoides Fox, and the Chrysidid Pseudolopyga carrilloi. Thanks to their studies, carried out in California and in laboratory, it turns out that the female Chrysidid places the egg on the living larva of the first or of the second stage of a Hemipteran bug of the Genus Nysius (Hemiptera Lygaeidae, two species being involved: N. raphanus and N. tenellus). The two species of Solierella use paralized Nysius larvas in order to provision the nest, 4-10 larvas per cell. In such a complicated way the Chrysidid is able to make its egg enter the nest of the host without being seen and without the risk of the adult host noticing its presence and destroying its egg. It is noticeable that the egg will develop only in the case that the larva that carries it is captured and paralyzed by the Solierella. That's one is the only known case of a Chrysidid linking its egg just on a free host, which will be used in a second time as a prey by a sphecid. An interesting case of competition against the Pseudolopyga comes from a sympatric species, Hedychridium solierellae, which parasites the same species of Solierella and the Pseudolopyga itself. This Chrysidid directly places its egg in the Solierella cell and its larva feeds on the host larva, on its supplies and also on the egg or on the larva of Pseudolopyga, when present.
Carrillo & Caltagirone (1970) have made detailed observations on the host-parasite relations between two sphecid species, Solierella peckhami (Ashmead) and S. plenoculoides Fox, and the Chrysidid Pseudolopyga carrilloi. Thanks to their studies, carried out in California and in laboratory, it turns out that the female Chrysidid places the egg on the living larva of the first or of the second stage of a Hemipteran bug of the Genus Nysius (Hemiptera Lygaeidae, two species being involved: N. raphanus and N. tenellus). The two species of Solierella use paralized Nysius larvas in order to provision the nest, 4-10 larvas per cell. In such a complicated way the Chrysidid is able to make its egg enter the nest of the host without being seen and without the risk of the adult host noticing its presence and destroying its egg. It is noticeable that the egg will develop only in the case that the larva that carries it is captured and paralyzed by the Solierella. That's one is the only known case of a Chrysidid linking its egg just on a free host, which will be used in a second time as a prey by a sphecid. An interesting case of competition against the Pseudolopyga comes from a sympatric species, Hedychridium solierellae, which parasites the same species of Solierella and the Pseudolopyga itself. This Chrysidid directly places its egg in the Solierella cell and its larva feeds on the host larva, on its supplies and also on the egg or on the larva of Pseudolopyga, when present.
